D2.3.1 Solvation with water as the solvent (or how things dissolve in water)
Water is a good solvent for polar compounds such as sodium chloride because water is a dipolar molecule where the 2 hydrogen atoms are positively charged and are mostly on one side, and the oxygen atom is negatively charged and on the other side.
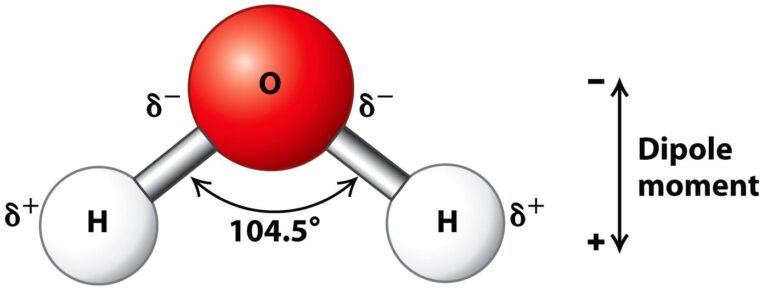
Summarise:
Water is a (1) molecule and therefore a good solvent for (2) compounds. When sodium chloride dissolves in water, the attractions between Sodium and the (3) atoms of water overcome the ionic atractions, causing the sodium to disperse. The attractions between chloride and the (4) atoms of water also overcome the ionic attractions, causing chloride to disperse.
Q) Discuss, why dont fatty acids dissolve readily in water, and does the length of a fatty acid chain affect this property?
D2.3.2 Water movement from less concentrated to more concentrated solutions
Water molecules are affected by attractions to polar solutes, such as sodium chloride or glucose.
The random movements of water molecules across a cell membrane by diffusion, are therefore also affected by water-solute attractions.
There are less movements away from the side of the membrane where there is more solute molecules, because these water-solute attractions tend to hold on to the water molecules.
Osmosis is therefore the net movement of water across a membrane to the side that has a higher concentration of solutes.
Keywords:
- hypotonic: a weaker solute concentration
- hypertonic: a stronger solute concentration
- isotonic: the same solute concentration
Osmosis is the therefore the movement of water molecules from h________ to _________, across a cell membrane.
D2.3.3 Water movement by osmosis into or out of cells
The plasma membrane is more permeable to water than to solutes. A difference in solute concentration across the plasma membrane of a cell, therefore results in a movement of water and not of solutes.
Osmosis is a passive movement. However, cells can manipulate osmotic pressure by using active transport to pump solutes into or out of cells.
Explain: The dispersion of squirting cucumber plants in terms of osmosis in the cells of plants.
Theorise about the mechanism of the closing leaves of the mimosa plant, using principles of osmosis.
D2.3.4 Changes due to water movement in plant tissues bathed in hypotonic and those bathed in hypertonic solutions. (How to measure the effect of osmosis in plants tissues).
The effect of osmosis on plant tissues can be quantitatively measured.
Plant tissues bathed in hypotonic solutions will gain mass and size. Plant tissues bathed in hypertonic solutions will lose mass and size.
Hints:
- You will need to provide a range of concentrations, some hypertonic and some hypotonic
- You will need samples of plants that are comparable or highly similar
- Make sure the surface of the plant tissues is dry before you start.
- Keep all other variables except for concentration, constant.
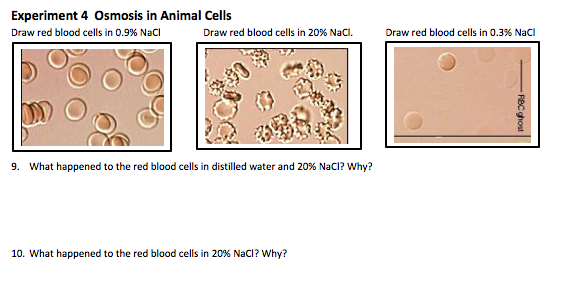
D2.3.5 Effects of water movement on cells that lack a cell wall (why animal cells burst of shrivel up in fresh and salt water respectively)
Without a cell wall, animal cells are vulnerable to the effects of osmosis.
A mammalian blood cell placed in a hypotonic solution will swell untill the membrane ruptures (lysis).
A mammalian blood cell placed in a hypertonic solution will shrivel, forming indentations in the membrane (crenations).
Discussion questions:
- Why are isotonic drinks desirable for marathon runners?
- Is drowning more deadly in fresh or salt water, and why?
D2.3.6 Effects of water movement n cells with a cell wall
Bacterial cells and plant cells are less vulnerable to the effects of osmosis because of the cell wall, which is a solid with a high tensile strength (cellulose in plants or peptidogylcan in bacteria).
Plant cells can become turgid (swollen) when places in hypotonic solutions, and flaccid (limp) when places in hypertonic solutions. The cytoplasm can pull away from the cell wall, something caused plasmolysis.
Because plants have no skeletal structures non-woody plants will become shrivelled if the cells are plasmolysed, and will stand upright if the cells are turgid.
D2.3.7 Medical applications of isotonic solutions
Isotonic solutions are highly important in medical procedures, as they keep human tissues osmotically stable and healthy.
Examples are:
- Eye drops (which are isotonic with 9g of NaCL per cubic decimetre)
- Saline fluid (used in the intravenous drips with 9g of NaCL per cubic decimetre)
- Organ donor bags (which contain isotonic solution in a preservation bag, surrouded by another semi-drozen isotonic solution.





