PART 1 CARBOHYDRATES
B1.1Chemical properties of a carbon atom allowing for the formation of diverse compounds on which life is based (or why carbon is special)
The chemicals that make up living organisms are based on carbon. Carbon is uniquely useful as an element for making biomolecules.
This is because:
- Carbon can make four covalent bonds. This allows for the formation of branching molecules with stable bonds.
- Carbon atoms can be joined together to make long chains of any length.
- Carbon chains of the right length can be folded into compact ring structures
Discussion question: Silicon is close to carbon in the periodic table, and also has a valency of four. Why isn´t life based on silicon instead of carbon?
B1.1.2 Production of macromolecules by condensation reactions to form a polymer (or take water, make a bond).
Throughout biochemistry, a reccurent theme occurs that small molecules called monomers are joined together to form big molecules called polymers.
Each time a bond is formed, water is eliminated.
This is called condensation.
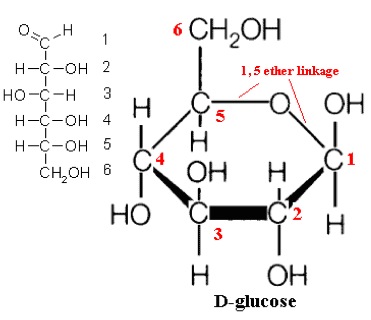
The image above shows Glucose (D-glucose is the form of glucose that exists in lifeforms on Earth). The carbon atoms in glucose each have a hydrogen, and a hydrogen and oxygen (a hydroxide group). The carbon atoms each have a number, 1-6. Glucose forms a ring by combining the carbons 1 and 5, losing the double bond with oxygen. This is a stable configuration.
The Human Brain is totally dependent on this molecule for survival. If the supply of Glucose is interrupted for as little as 15minutes, brain cells start to die. No other organ in the body is totally dependent on glucose like this.
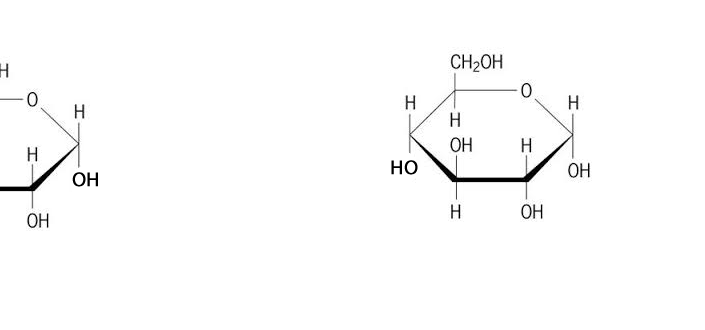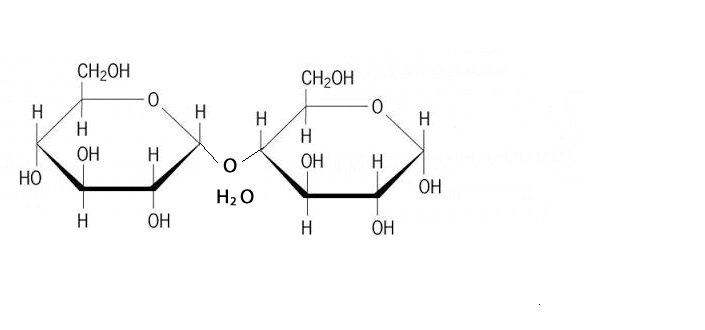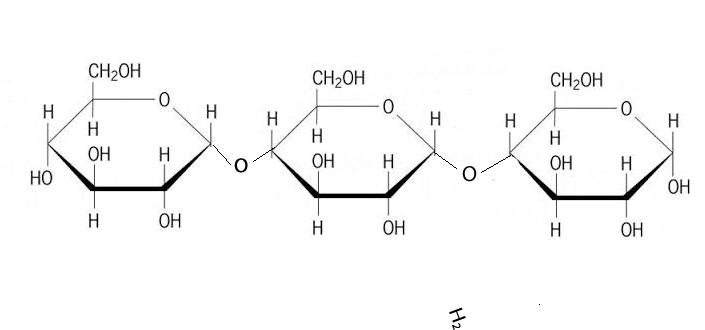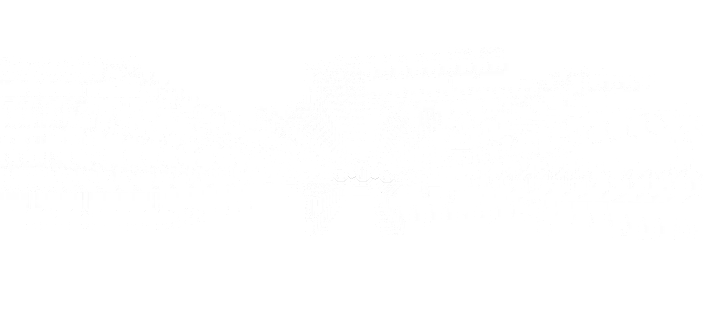
Discussion: Observe the GIF above. Try to explain as specifically as you can what is happening.
Summary of condensation: Take water, make a bond
B1.1.3 Digestion of polymers into monomers by hydrolysis reactions.
The large biomolecules called polymers are often broken down and reconstructed in a different configuration to make other molecules.
Sometimes they are broken down, and then used as a source of energy in respiration.
The opposite reaction for condensation adds water back to the bond, breaking it down in the process. This is called hydrolysis.
The summary of hydrolysis: Add water, break a bond.
Discussion: If a starch molecule is made up of 400 glucose molecules (it would be a kind called alpha glucose), how many molecules of water would be required to break it down into individual glucose molecules?
B1.1.4 Form and function of monosaccharides (or why are sugars made from one ring so necessary)
A monosaccharide is a sugar made from a single ring of carbon atoms joined through an oxygen. They are also called ´single sugars´.
Glucose is the most famous monosaccharide. Galactose(6 carbons), fructose (6 carbons) and deoxyribose (5 carbons) are other monosaccharides.
Monosaccharides are useful because:
- They are small and soluble, so easy to move around in the body.
- They do not need to be broken down much so they can be used in respiration to gain energy quickly
Discussion: Why do we like the taste of sugars so much, especially when we are young?
B1.1.5 Polysaccharides as energy storage compounds (or how starches can store energy)
Polysaccharides are basically polymers of monosaccharides. For example starch is made of long chains of glucose.
There are two main kinds of Glucose:
- Alpha Glucose
- Beta Glucose
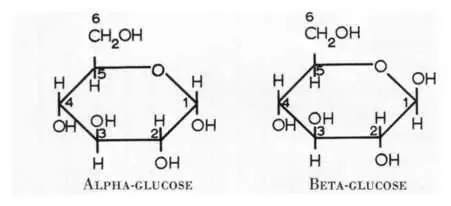
Discussion: The chart above shows Alpha and Beta glucose. What is the difference?
Glucose can be joined together to make polysaccharides in two ways:
- 1-4 Glycosidic bonds, formed by joining carbons 1 and 4 on adjacent glucose molecules. This leads to straight chains of glucose molecules.
- 1-6 Glycosidic bonds, formed by joining carbons 1 and 6 on adjacent glucose molecules. This leads to branched chains of glucose molecules.
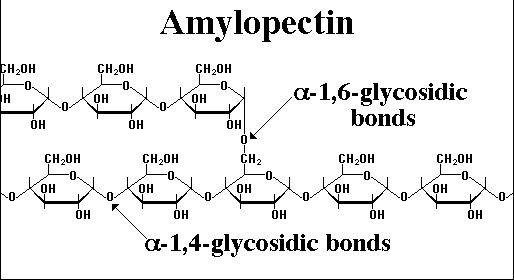
Discussion: What can the relative advantages of 1-4 and 1-6 glycosidic bonds.
B1.1.6 Structure of cellulose related to its function as a structural polysaccharride in plants (why cellulose is not for energy)
Cellulose is made of long straight chains of glucose (polysaccharides). It has a a structural function as the chief component of cell walls
Discuss: What is the link between the structure of cellulose and the kinds of bonds.
Note: Cellulose is made from Beta glucose. This means it cannot be digested by the human digestive system.
B1.1.7 Role of Glycoproteins in cell-cell recognition (or why the glycoproteins we learned in cell membranes explains blood types).
Discussion questions:
- How does this connect with what we learned about glycoproteins when we studied cell membranes?
- How is blood type O different to blood types A and B?
Section summary:
- Blood types are caused by glycoproteins embedded in the cell membranes of red blood cells.
- Type A and Type B are both glycoproteins. In the context of immunity, they are also referred to as antigens because they can trigger an immune response.
- Blood type O do not have either the A or B antigen.
- Blood type AB have both A and B antigens present on their red blood cells
- There is another blood type system Rhesus + and -.
- Rhesus + people have another antigen called D or Rhesus antigen, present on their red blood cells.
- Rhesus – people do not have Rhesus or the D antigen present on their red blood cells
Discuss: Explain your own blood type in terms of glycoproteins?
B1.1.8 Hydrophobic properties of lipids (a quick summary of the different kinds of lipids)
Lipids are a diverse group of biomolecules, which contain Carbon, Hydrogen and Oxygen like carbohydrates, but significantly less Oxygen.
This causes them to by hydrophobic. This means they are more attracted to non-polar substances, than polar ones.
There are three main types of lipids:
- Triglycerides. These all have a similar structure consisting of three fatty acid chains joined through a molecule of glycerol. Oils, fats, and waxes are all classes of triglycerides with differing melting points.
- Free fatty acids. Fatty acids may exist independently of triglycerides.
- Steroids. These have a very different structure based on four rings. All the sex hormones, and cortisol (the stress hornone) are examples of steroids.
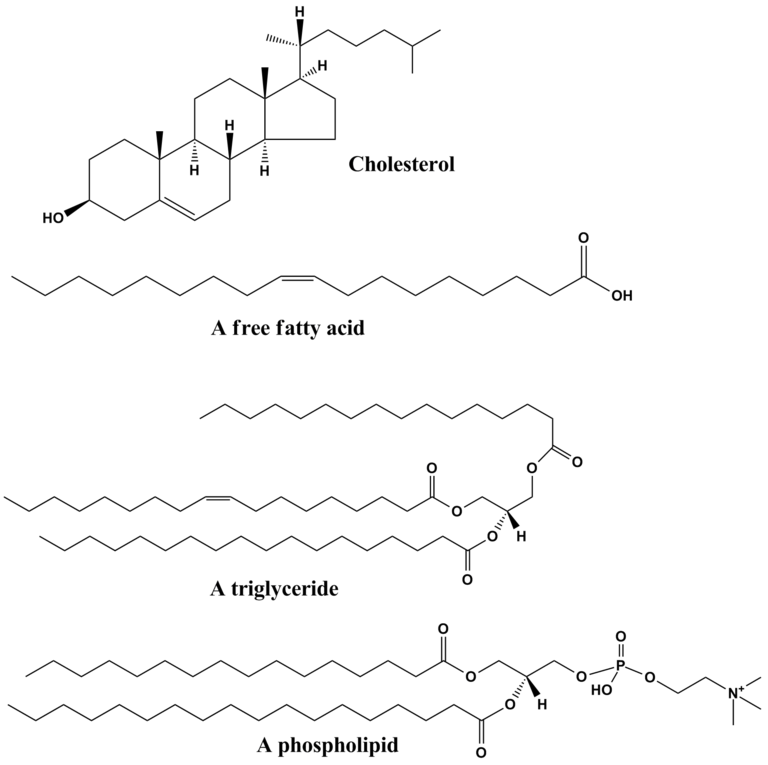
Discuss: Which kinds of lipids are more closely related to each other?
B1.1.9 Formation of triglycerides and phospholipids by condensation reactions (or how you can join glycerol and three fatty acids by removing 3 molecules of water).
Discussion question: How many condensation reactions would you expect in the formation of a phospholipid?
B1.1.10 Difference between monosaturated and polyunsaturated fatty acids (why it is all about the fatty acid chains)
The fatty acid chains in different triglycerides can vary greatly in size and also number of double bonds.
Varying the size affects mostly the melting point of triglycerides. For example oils which are liquid at room temperature, tend to consist of shorter fatty acid chains than fats, which are solid at room temperature.
The Double bonds in the fatty acid chains account for great differences in the health implications of fatty acids.
For example:
- Saturated fatty acid chains have no double bonds at all. These are considered to be harmful for your health as there is a correlation between their consumption and the incidence of coronary heart disease.
- Monosaturated fatty acid chains have one double bond. These are considered relatively health. The position of this double bond can make a big difference eg. Omega 3 linolenic acid is a fatty acid you MUST have in your diet to stay healthy. It´s called Omega 3 because the double bond is the third bond away from the end of the fatty acid (the CH3 or methyl group on the end).
- Polyunsaturated fatty acid chains have more than one double bond. These are also considered relatively healthy.
Some fatty acids are straight chains, and others have kinks or bends in the chains.
The more space a fatty acid occupies, the harder it is to move around the body.
What is the cause of the straight or angled fatty acid chains?
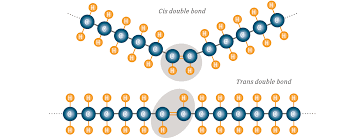
What is the cause of the straight or angled fatty acid chains? Each double bond has a pair of hydrogen atoms on either side of the double bond. These repel each other.
So if the hydrogens are on the same side (cis), this causes the fatty acid chain to bend at an angle
If the hydrogens are on opposite sides (trans), the chain remains straight and does not bend.
Discussion: Observe the two fatty acids above. What can you infer about their structures, and about their likely impact on human health in the diet?
B1.1.11 Triglycerides in adipose tissues for energy storage and thermal insulation (Why % body fat is not always such a bad idea)
Triglycerides are used for energy storage in animals and plants. Unlike carbohydrates, the energy in lipids may take days to become available as it needs to be broken down slowly. Adipose tissue, or specialised cells underneath the skin that store fats, are an adaptation to situations where no food is available.
However, twice as much energy can be gained from the same mass of lipids, as from carbohydrates. Fats are also stable, and hydrophobic so they do not affect the osmotic balance of a cell.
Fat stored under the skin has the aided bonus of acting as a thermal insulator.
Fat stored around major organs such as the kidneys acts as a natural shock absorber, protecting vital organs.

Discussion: Male emperor penguins will fast for 115 days while they find a mate, breed, and then lovingly guard the egg until it hatches, waiting for their female mate to returnfrom an extended fishing trip in the Antartic seas. During this time they may only move a few feet, keeping the precious egg balanced on their toes. How does adipose tissue help them to achieve this remarkable feat of parenting?
B1.1.12 Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions (how phospholipids are held together).
The phospholipid bilayer consists of a double layer, where the hydrophobic fatty acid chains point towards each other, away from the water which surrouds the membrane. The hydrophilic heads, containing the polar phosphate groups, point outwards towards the water.
This is the basis for cell membrane structure.
B1.1.13 Ability of non-polar steroids to cross the phospholipid bilayer (or why sex hormones do not need a protein channel to enter a cell).
As all steroids are lipids and therefore non-polar, they may cross the phopholipid bilayer without the use of a protein channel.
This makes it easier for them to affect many parts of the body.
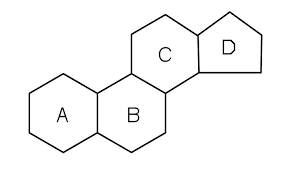
Discussion: Anabolic steroids are artificial steroids which often replicate almost exactly the structure of sex hormones, especially testosterone. Why is it that some body builders, male or female, sometimes take anabolic steroids?





